Natural Phenolic Compounds Targeting The AMPK Activation For Metabolic Health
- Articles
- Submited: April 7, 2021
-
Published: April 7, 2021
Abstract
AMP-activated protein kinase (AMPK) is a cellular energy sensor which plays a crucial role in regulation of whole-body energy homeostasis. Activation of AMPK signaling results in favorable effects on mitochondrial function, autophagy, glucose/lipid metabolism, and insulin sensitivity, making it an important therapeutic target in treatment/prevention of metabolic disorders and cancer. Recently, pharmacological studies of natural phenolic compounds indicated that the benefits on metabolic health of these phytochemicals are not only related to their protogenic antioxidant property but also to their AMPK-activating potential. Due to their diverse structures, identification of phenolic compound molecules which have potential to target the AMPK activation for beneficial metabolic effects may be promising in order to develop novel therapeutics in the prevention and/or treatment of metabolic disorders. In this minireview, we summarize beneficial metabolic outcomes of AMPK activation and discuss the capability of natural polyphenols to activate the AMPK pathway focusing on the phenolic acids as potential lead compounds.
References
- David Moller. New drug targets for type 2 diabetes and the metabolic syndrome. Nature, 414:821–7, 01 2002.
- David Hardie and Michael Ashford. Ampk: Regulating energy balance at the cellular and whole body levels. Physiology (Bethesda, Md.), 29:99–107, 03 2014.
- Hayley O’Neill, Graham Holloway, and Gregory Steinberg. Ampk regulation of fatty acid metabolism and mitochondrial biogenesis: Implications for obesity. Molecular and cellular endocrinology, 366, 06 2012.
- Kimberly Coughlan, Rudy Valentine, Neil Ruderman, and Asish Saha. Ampk activation: A therapeutic target for type 2 diabetes? Diabetes, metabolic syndrome and obesity : targets and therapy, 7:241–53, 06 2014.
- David Hardie. Regulation of amp-activated protein kinase by natural and synthetic activators. Acta Pharmaceutica Sinica B, 20, 07 2015.
- David Stevenson and Roger Hurst. Polyphenolic phytochemicals - just antioxidants or much more? Cellular and molecular life sciences : CMLS, 64:2900–16, 12 2007.
- Duangjai Tungmunnithum, Areeya Thongboonyou, Apinan Pholboon, and Aujana Yangsabai. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines (Basel, Switzerland), 5(3):93, Aug 2018. 30149600[pmid].
- Shu Wang, Naima Moustaid-Moussa, Lixia Chen, Huanbiao Mo, AnuradhaShastri, RuiSu, PriyankaBapat,InsookKwun, and Chwan-Li Shen. Novel insights of dietary polyphenols and obesity. The Journal of nutritional biochemistry, 25:1–18, 01 2014.
- David Hardie. Hardie, d. g. amp-activated/snf1 protein kinases: Conserved guardians of cellular energy. nature rev. mol. cell biol. 8, 774-785. Nature reviews. Molecular cell biology, 8:774–85, 11 2007.
- David Hardie, Fiona Ross, and Simon Hawley. Ampk: A nutrient and energy sensor that maintains energy homeostasis. Nature reviews. Molecular cell biology, 13:251–62, 03 2012.
- David Hardie. Ampk: A target for drugs and natural products with effects on both diabetes and cancer. Diabetes, 62:2164– 2172, 07 2013.
- David Hardie. Amp-activated proteinkinase: Akeyregulator of energy balance with many roles in human disease. Journal of Internal Medicine, 276, 05 2014.
- Morgan Fullerton, Sandra Galic, Katarina Marcinko, Sarah Sikkema, Thomas Pulinilkunnil, Zhi-Ping Chen, Hayley O’Neill, Rebecca J Ford, Rengasamy Palanivel, Matthew O’Brien, David Hardie, Stuart Macaulay, Jonathan Schertzer, JasonDyck, BryceDenderen, BruceKemp,andGregorySteinberg. Single phosphorylation sites in acc1 and acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nature medicine, 19, 11 2013.
- Sibylle Jäger, Christoph Handschin, Julie St-Pierre, and Bruce Spiegelman. Jager s, handschin c, st-pierre j, spiegelman bm.. amp-activated proteinkinase (ampk) action in skeletal muscle via direct phosphorylation of pgc-1alpha. proc natl acad sci usa 104: 12017-12022. Proceedings of the National Academy of Sciences of the United States of America, 104:12017–22, 08 2007.
- Jiandie Lin, Christoph Handschin, and Bruce M Spiegelman. Metabolic control through the pgc-1 family of transcription coactivators. Cell metabolism, 1(6):361—370, June 2005.
- David Hardie. Amp-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes development, 25:1895–908, 09 2011.
- Carles Cantó and Johan Auwerx. Pgc-1α, sirt1 and ampk, an energy sensing network that controls energy expenditure. Current opinion in lipidology, 20:98–105, 05 2009.
- Marie Lagouge, Carmen Argmann, Zachary Gerhart-Hines, Hamid Meziane, Carles Lerin, Frederic Daussin, Nadia Messadeq, Jill Milne, Philip Lambert, Peter Elliott, Bernard Geny, Markku Laakso, Pere Puigserver, and Johan Auwerx. Resveratrol improves mitochondrial function and protects against metabolic disease by activating sirt1 and pgc-1α. Cell, 127:1109–22, 01 2007.
- Marcella Fulco, Yana Cen, Po Zhao, Eric P Hoffman, Michael W McBurney, Anthony A Sauve, and Vittorio Sartorelli. Glucose restriction inhibits skeletal myoblast differen
- tiation by activating sirt1 through ampk-mediated regulation of nampt. Developmental cell, 14(5):661—673, May 2008.
- Carles Cantó, Zachary Gerhart-Hines, Jerome Feige, Marie Lagouge, Lilia Noriega, Jill Milne, Peter Elliott, Pere Puigserver, and Johan Auwerx. Ampk regulates energy expenditure by modulating nad+ metabolism and sirt1 activity. Nature, 458:1056–60, 04 2009.
- Daniel Klionsky, Fabio Abdalla, Hagai Abeliovich, Robert Abraham, Abraham Acevedo-Arozena, Khosrow Adeli, Lotta Agholme, Maria Agnello, Patrizia Agostinis, Julio AguirreGhiso, Hyung, Jun Ahn, Ouardia Ait-Mohamed, Slimane AitSi-Ali,TakahikoAkematsu,ShizuoAkira,HeshamAl-Younes, MunirAl-Zeer,MatthewAlbert,andBehzadYeganeh. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy, 4454:445–544, 04 2012.
- Yuchen Feng, Ding He, Zhiyuan Yao, and Daniel Klionsky. The machinery of macroautophagy. Cell research, 24, 12 2013.
- Joungmok Kim, Mondira Kundu, Benoit Viollet, and KunLiang Guan. Ampk and mtor regulate autophagy via direct phosphorylation of ulk1. Nature cell biology, 13:132–41, 02 2011.
- Daniel Egan, Joungmok Kim, Reuben Shaw, and Kun-Liang Guan. The autophagy initiating kinase ulk1 is regulated via opposing phosphorylation by ampk and mtor. Autophagy, 7:643–4, 06 2011.
- Rajat Singh, Susmita Kaushik, Yongjun Wang, Youqing Xiang, Inna Novak, Masaaki Komatsu, Keiji Tanaka, Ana Maria Cuervo, and Mark J. Czaja. Autophagy regulates lipid metabolism. Nature, 458(7242):1131–1135, April 2009.
- Zheng dh, Paul MacLean, Steven Pohnert, John Knight, Ann Olson, William Winder, and G. Dohm. Regulation of muscle glut-4 transcription by amp-activated protein kinase. Journal of applied physiology (Bethesda, Md. : 1985), 91:1073–83, 10 2001.
- Kay Barnes, Jean C Ingram, Omar H Porras, L Felipe Barros, Emma R Hudson, Lee G D Fryer, Fabienne Foufelle, David Carling, D Grahame Hardie, and Stephen A Baldwin. Activation of glut1 by metabolic and osmotic stress: potential involvement of amp-activated protein kinase (ampk). Journal of cell science, 115(Pt 11):2433—2442, June 2002.
- Bradford Lowell and Gerald Shulman. Mitochondrial dysfunctionandtype2diabetes. Science(NewYork,N.Y.),307:384– 7, 02 2005.
- Marc Liesa and Orian Shirihai. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell metabolism, 17:491–506, 04 2013.
- ErikARichterandNeilBRuderman. Ampkandthebiochemistry of exercise: implications for human health and disease. The Biochemical journal, 418(2):261—275, March 2009.
- Maria Mihaylova and Reuben Shaw. The ampk signaling pathway coordinates cell growth, autophagy and metabolism. Nature cell biology, 13:1016–23, 09 2011.
- Mark OWEN, Elena DORAN, and Andrew HALESTRAP. Evidence that metformin exerts its anti-diabetic effects through inhibitionofcomplex1ofthemitochondrialrespiratorychain. The Biochemical journal, 348 Pt 3:607–14, 07 2000.
- MY El-Mir, V Nogueira, E Fontaine, N Avéret, M Rigoulet, and X Leverve. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex i. The Journal of biological chemistry, 275(1):223—228, January 2000.
- Guangqian Zhou, Robert Myers, Ying Li, Yuli Chen, Xiaolan Shen, JudyFenyk-Melody, MargaretWu, JohnVentre, Thomas Doebber, Nobuharu Fujii, Nicolas Musi, Michael Hirshman, Laurie Goodyear, and David Moller. Role of amp-activated protein kinase in mechanism of metformin action. The Journal of clinical investigation, 108:1167–74, 11 2001.
- Simon Hawley, Fiona Ross, Cyrille Chevtzoff, Kevin Green, Ashleigh Philp, Sarah Fogarty, Mhairi Towler, Laura Brown, Oluseye Ogunbayo, A Evans, and David Hardie. Use of cells expressing γ sub unit variants to identify diverse mechanisms of ampk activation. Cell metabolism, 11:554–65, 06 2010.
- Yong Deuk Kim, Keun-Gyu Park, Yong-Soo Lee, Yun-Yong Park, Don-Kyu Kim, Balachandar Nedumaran, Won Gu Jang, Won-Jea Cho, Joohun Ha, In-Kyu Lee, Chul-Ho Lee, and Hueng-Sik Choi. Metformin inhibits hepatic gluconeogenesis through amp-activated protein kinase–dependent regulation of the orphan nuclear receptor shp. Diabetes, 57(2):306–314, 2008.
- Russell Miller, Qingwei Chu, Jianxin Xie, Marc Foretz, Benoit Viollet, and Morris Birnbaum. Biguanides suppress hepatic glucagon signaling by decreasing production of cyclic amp. Nature, 494, 01 2013.
- Lee Fryer, Asha Parbu-Patel, and David Carling. The antidiabetic drugs rosiglitazone and metformin stimulate AMP activated protein kinase through distinct signaling pathways. The Journal of biological chemistry, 277:25226–32, 08 2002.
- Barbara Brunmaier, Katrin Staniek, Florian Gras, Nicole Scharf, Aleksandra Althaym, Renate Clara, Michael Roden, Erich Gnaiger, Hans Nohl, Werner Waldhäusl, and Clemens Fürnsinn. Thiazolidinediones, like metformin, inhibit respiratory complex i: A common mechanism contributing to their antidiabetic actions? Diabetes, 53:1052–9, 04 2004.
- Tetsuya Kubota Hiroki Kumagai Shinsuke Itoh Hidemi Satoh Wataru Yano Hitomi Ogata Kumpei Tokuyama Iseki TakamotoTomokaMineyamaMichiroIshikawaMasaoMoroi KaoruSugiToshimasaYamauchiKohjiroUekiKazuyukiTobe Tetsuo Noda Ryozo Nagai Takashi Kadowaki Naoto Kubota, Yasuo Terauchi. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. TheJournalofBiologicalChemistry281, 8748-8755, 01 2006.
- Masato Iwabu, Toshimasa Yamauchi, Miki Okada-Iwabu, Koji Sato, Tatsuro Nakagawa, Masaaki Funata, Mamiko Yamaguchi, Shigeyuki Namiki, Ryo Nakayama, Mitsuhisa Tabata, Hitomi Ogata, Naoto Kubota, Iseki Takamoto, Yukiko Hayashi, Naoko Yamauchi, Hironori Waki, Masashi Fukayama, Ichizo Nishino, Kumpei Tokuyama, and Takashi Kadowaki. Adiponectin and adipor1 regulate pgc-1 and mitochondria by ca2+ and ampk/sirt1. Nature, 464:1313–9, 03 2010.
- Jonathan R. Gledhill, Martin G. Montgomery, Andrew G. W. Leslie, and John E.Walker. Mechanismofinhibitionofbovine f1-ATPase by resveratrol and related polyphenols. Proceedings of the National Academy of Sciences, 104(34):13632–13637, 2007.
- Nigel Turner, Jing-Ya Li, Alison Gosby, Sabrina W.C. To, Zhe Cheng, Hiroyuki Miyoshi, Makoto M. Taketo, Gregory J. Cooney, Edward W. Kraegen, David E. James, Li-Hong Hu, Jia Li, and Ji-Ming Ye. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex i. Diabetes, 57(5):1414–1418, 2008.
- Joseph Baur and David Sinclair. Therapeutic potential of resveratrol: The in vivo evidence. Nature reviews. Drug discovery, 5:493–506, 07 2006.
- Jee-Hyun Um, Sung-Jun Park, Hyeog Kang, Shutong Yang,
- Marc Foretz, Michael W. McBurney, Myung K. Kim, Benoit Viollet, and Jay H. Chung. Amp-activated protein kinase–deficient mice are resistant to the metabolic effects of resveratrol. Diabetes, 59(3):554–563, 2010.
- Nathan Price, Ana Gomes, Alvin J.Y. Ling, Filipe Duarte, Alejandro Martin-Montalvo, Brian North, Beamon Agarwal, Lan Ye, Giorgio Ramadori, João Teodoro, Basil Hubbard, Ana Varela, James Davis, Behzad Varamini, Angela Hafner, Ruin Moaddel, Anabela Rolo, Roberto Coppari, Carlos Palmeira, and David Sinclair. Sirt1 is required for ampk activation and the beneficial effects of resveratrol on mitochondrial function. Cell metabolism, 15:675–90, 05 2012.
- Xiuyun Hou, Shanqin Xu, Karlene Maitland-Toolan, Kaori Sato, Bingbing Jiang, Yasuo Ido, Fan Lan, Kenneth Walsh, Michel Wierzbicki, Tony Verbeuren, Richard Cohen, and Mengwei Zang. Sirt1 regulates hepatocyte lipid metabolism through activating amp-activated protein kinase. The Journal of biological chemistry, 283:20015–26, 08 2008.
- Joseph Baur, Kevin Pearson, Nathan Price, Hamish Jamieson, Carles Lerin, Avash Kalra, Vinayakumar Prabhu, Joanne Allard, Guillermo Lopez-Lluch, Kaitlyn Lewis, Paul Pistell, Suresh Poosala, Kevin Becker, Olivier Boss, Dana Gwinn, MingyiWang, Sharan Ramaswamy, Kenneth Fishbein, Richard Spencer, and David Sinclair. Resveratrol improves health and survival of mice on a high-calorie diet. Nature, 444:337–342, 11 2006.
- Silvie Timmers, Ellen Konings, Lena Bilet, Riekelt Houtkooper, Tineke van de Weijer, Gijs Goossens, Joris Hoeks, Sophie Krieken, Dongryeol Ryu, Sander Kersten, Esther Moonen-Kornips, Matthijs Hesselink, Iris Kunz, Vera Schrauwen-Hinderling, Ellen Blaak, Johan Auwerx, and Patrick Schrauwen. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell metabolism, 14:612–22, 11 2011.
- Sanne van der Made, Jogchum Plat, and Ronald Mensink. Resveratrol does not influence metabolic risk markers related to cardiovascular health in overweight and slightly obese subjects: A randomized, placebo-controlled crossover trial. PloS one, 10:e0118393, 03 2015.
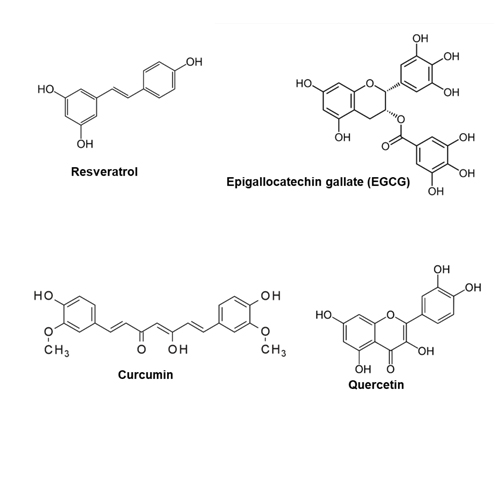
- Yueshui Zhao, Bo Chen, Jing Shen, Lin Wan, Yinxin Zhu, and Zhangang Xiao. The beneficial effects of quercetin, curcumin, and resveratrol in obesity. Oxidative Medicine and Cellular Longevity, 2017:1–8, 08 2017.
- Catalina Carrasco-Pozo, María Cires, and Martin Gotteland. Quercetin and epigallocatechin gallate in the prevention and treatment of obesity: From molecular to clinical studies. Journal of Medicinal Food, 22, 05 2019.
- Qu Collins, Hui-Yu Liu, Jingbo Pi, Zhenqi Liu, Michael Quon, and Wenhong Cao. Epigallocatechin-3-gallate (egcg), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5’-amp-activated protein kinase. The Journal of biological chemistry, 282:30143–9, 10 2007.
- Kejing Zeng, Lili Tian, Rucha Patel, Weijuan Shao, Zhuolun Song, Ling Liu, Justin Manuel, Max Ma, Ian McGilvray, Carolyn Cummins, Jianping Weng, and Tianru Jin. Diet polyphenolcurcuminstimulateshepaticfgf21productionandrestores its sensitivity in high-fat-diet-fed male mice. Endocrinology, 158:jc.2016.1596, 12 2016.
- Jin Zhou, Benjamin Farah, Rohit Sinha, Yajun Wu, Brijesh Singh, Boon-Huat Bay, Chung Yang, and Paul Yen. Epigallocatechin-3-gallate (egcg), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PloS one, 9:e87161, 01 2014.
- LiangLiu,ChaoGao,PingYao,andZhiyongGong. Quercetin alleviates high-fat diet-induced oxidized low-density lipoprotein accumulation in the liver: Implication for autophagy regulation. BioMed Research International, 2015:1–9, 12 2015.
- Naresh Kumar and Nidhi Goel. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnology reports (Amsterdam, Netherlands), 24:e00370– e00370, Aug 2019. 31516850[pmid].
- Gregory R. Steinberg, Madhumita Dandapani, and D. Grahame Hardie. Ampk: mediating the metabolic effects of salicylate-baseddrugs? TrendsinEndocrinologyandMetabolism, 24(10):481–487,October2013. G.R.S.issupportedbyaCanada Research Chair in Metabolism and Obesity and grants from the Canadian Institutes of Health Research and the Canadian Diabetes Association. D.G.H. is supported by a Senior Investigator Award from the Wellcome Trust and a Programme Grant from Cancer Research UK, and by the pharmaceutical companies supporting the Division of Signal Transduction Therapy at Dundee (AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck KgaA, Janssen Pharmaceutica, and Pfizer). M.D. was supported by a Clinical PhD studentship from the Wellcome Trust. Commissioned review article.
- JR Vane. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature: New biology, 231(25):232—235, June 1971.
- E Kopp and S Ghosh. Inhibition of nf-kappa b by sodium salicylate and aspirin. Science (New York, N.Y.), 265(5174):956—959, August 1994.
- Carmen Montesinos and Gerald Weissmann. Salicylates and sulfasalazine, but not glucocorticoids, inhibit leukocyte accumulation by an adenosine-dependent mechanism that is independentofinhibitionofprostaglandinsynthesisandp105 of nfkappa b. Proceedings of the National Academy of Sciences, 96, 06 1999.
- Simon A. Hawley, Morgan D. Fullerton, Fiona A. Ross, Jonathan D. Schertzer, Cyrille Chevtzoff, Katherine J. Walker, Mark W. Peggie, Darya Zibrova, Kevin A. Green, Kirsty J. Mustard, Bruce E. Kemp, Kei Sakamoto, Gregory R. Steinberg, and D. Grahame Hardie. The ancient drug salicylate directly activates amp-activated protein kinase. Science (New York, N.Y.), 336(6083):918–922, May 2012. 22517326[pmid]. [63] Andrew Obrien, Linda Villani, Lindsay Broadfield, Vanessa Houde, Sandra Galic, Giovanni Blandino, Bruce Kemp, Theodoros Tsakiridis, Paola Muti, and Gregory Steinberg. Salicylate activates ampk and synergizes with metformin to reduce the survival of prostate and lung cancer cells ex vivo through inhibition of de novo lipogenesis. The Biochemical journal, 469, 05 2015.
- Lin Han, Qing Yang, Jia Li, feier cheng, Yao Zhang, Yunlong Li, and Min Wang. Protocatechuic acid-ameliorated endothelial oxidative stress through regulating acetylation level via cd36/ampk pathway. Journal of Agricultural and Food Chemistry, 67, 06 2019.
- Yunu Jung, Jinbong Park, Hye-Lin Kim, Jung-Eun Sim, DongHyun Youn, Jongwook Kang, Seona Lim, Mi-Young Jeong, WoongMoYang,Seok-GeunLee,KwangAhn,andJae-Young Um. Vanillic acid attenuates obesity via activation of the ampk pathway and thermogenic factors in vivo and in vitro. The FASEB Journal, 32:fj.201700231RR, 11 2017.
- Miori Tanaka, Akari Sato, Yoshimi Kishimoto, Hideaki
- Mabashi-Asazuma, Kazuo Kondo, and Kaoruko Iida. Gallic acid inhibits lipid accumulation via ampk pathway and suppresses apoptosis and macrophage-mediated inflammation in hepatocytes. Nutrients, 12(5), May 2020.
- Khanh Doan, Chang Ko, Ann Kinyua, Dong Yang, Yun-Hee Choi, In Oh, Nguyen Nguyen, Ara Ko, Jae Choi, Yangsik Jeong,MinJung,WonCho,ShanhuaXu,Kyu-SangPark,Woo Park, Soo Choi, Hyoung Kim, Sang Hyun Moh, and Ki Kim. Gallic acid regulates body weight and glucose homeostasis through ampk activation. Endocrinology, 156:en20141354, 10 2014.
- En-Pei Chiang, Shu-Yao Tsai, Yueh-Hsiung Kuo, Man-Hui Pai, Hsi-Lin Chiu, Raymond Rodriguez, and Feng-Yao Tang. Caffeic acid derivatives inhibit the growth of colon cancer: Involvement of the pi3-k/akt and ampk signaling pathways. PloS one, 9:e99631, 06 2014.
- Rafaela Scalco Ferreira, Neife Santos, Carolina Bernardes, Flávia Malvestio Sisti, Lilian Amaral, Andréia Fontana, and Antonio Santos. Caffeic acid phenethyl ester (cape) protects pc12 cells against cisplatin-induced neurotoxicity by activating the ampk/sirt1, mapk/erk, and pi3k/akt signaling pathways. Neurotoxicity Research, 36, 04 2019.
- Eun Lee, Kyung-Ok Uhm, Yun Lee, Myoung Sook Han, MyungSik Lee, Ji-Man Park, Pann-Ghill Suh, Sun-Hwa Park, and Hyeon Kim. Cape (caffeic acid phenethyl ester) stimulates glucose uptake through ampk (amp-activated protein kinase) activation in skeletal muscle cells. Biochemical and biophysical research communications, 361:854–8, 11 2007.
- Hoda Eid, Farah Thong, Abir Nachar, and Pierre Haddad. Caffeic acid methyl and ethyl esters exert potential antidiabetic effects on glucose and lipid metabolism in cultured murine insulin-sensitive cells through mechanisms implicating activation of ampk. Pharmaceutical Biology, 55:2026–2034, 12 2017.
- Xiaoling Chen, Yafei Guo, Gang Jia, Hua Zhao, Guangmang Liu, and Zhiqing Huang. Ferulic acid regulates muscle fiber type formation through sirt1/ampk signaling pathway. Food Function, 10, 11 2018.
- Seung-WooKang, Seong-IlKang, Hye-SunShin, Seon-AYoon, Jeong-Hwan Kim, Hee-Chul Ko, and Se-Jae Kim. Sasa quelpaertensis nakai extract and its constituent p-coumaric acid inhibit adipogenesis in 3t3-l1 cells through activation of the ampk pathway. Foodand chemicaltoxicology: an international journal published for the British Industrial Biological Research Association, 59, 06 2013.